Marc Bardou, Alan N Barkun, Myriam MartelDisclosures
Gut. 2013;62(6):933-947.
Abstract
Excess body weight, as defined by the body mass index (BMI), has been associated with several diseases and includes subjects who are overweight (BMI≥25–29.9 kg/m2) or obese (BMI≥30 kg/m2). Overweight and obesity constitute the fifth leading risk for overall mortality, accounting for at least 2.8 million adult deaths each year. In addition around 11% of colorectal cancer (CRC) cases have been attributed to overweight and obesity in Europe. Epidemiological data suggest that obesity is associated with a 30–70% increased risk of colon cancer in men, whereas the association is less consistent in women. Similar trends exist for colorectal adenoma, although the risk appears lower. Visceral fat, or abdominal obesity, seems to be of greater concern than subcutaneous fat obesity, and any 1 kg/m2 increase in BMI confers additional risk (HR 1.03). Obesity might be associated with worse cancer outcomes, such as recurrence of the primary cancer or mortality. Several factors, including reduced sensitivity to antiangiogenic-therapeutic regimens, might explain these differences. Except for wound infection, obesity has no significant impact on surgical procedures. The underlying mechanisms linking obesity to CRC are still a matter of debate, but metabolic syndrome, insulin resistance and modifications in levels of adipocytokines seem to be of great importance. Other biological factors such as the gut microbita or bile acids are emerging. Many questions still remain unanswered: should preventive strategies specifically target obese patients? Is the risk of cancer great enough to propose prophylactic bariatric surgery in certain patients with obesity?
Introduction
According to the WHO, overweight and obesity are defined as abnormal or excessive fat accumulation in adipose tissue that may impair health (http://www.who.int/mediacentre/factsheets/fs311/en/). The WHO definition for overweight is a body mass index (BMI, weight/(height in m)2) ≥ 25 kg/m2 and for obesity BMI≥30 kg/m2. In 2008, 1.5 billion adults of 20 years and older were overweight. Of these, over 200 million men and nearly 300 million women were obese. The US 2009–2010 National Health and Nutrition Examination Survey showed an age-adjusted mean BMI of 28.7 (95% CI 28.3 to 29.1) for men and also 28.7 (95% CI 28.4 to 29.0) for women, and an adult obesity prevalence of 35.5% in men and 35.8% among women—unchanged from 2003–2008.[1] Obesity prevalence in US children and adolescents was 16.9%, unchanged from 2007–2008.[2] A study conducted in Australia, New Zealand and the UK in working nurses and midwives found that 62% were outside the healthy weight range.[3] In contrast, despite an increase over the past 30 years, obesity is half as common in France as in the USA or the UK (14.5% in 2009).[4] It is 10.1% among women and 11.4% among men in China.[5] As fat is principally deposited in two compartments, the subcutaneous and visceral compartments, it is interesting to note in this last study an associated increased prevalence of abdominal obesity (27.8% of men and 45.9% of women).[6] Only about two-thirds of patients with the metabolic syndrome (MS) are obese.[7,8] In contrast, some obese subjects are metabolically healthy.[9] MS is key linking obesity to most of its related complications such as diabetes, cardiovascular diseases or an increased risk of cancer in different sites, but may vary across different ethnic groups. The accumulation of ectopic fat, for example, in skeletal muscle and liver, might be strongly associated with MS,[9] whereas subcutaneous fat might be protective.[10] Recommendation to measure waist circumference and waist-to-height or waist-to-hip ratios rather than BMI recognises the important role played by abdominal obesity in MS, and the fact that BMI might overestimate obesity.[11]The publication of specific waist circumference cut-offs to define abdominal obesity is not supported by solid epidemiological and metabolic data;[12,13] furthermore ethnicity alters the relationship between visceral fat and MS.[14]
Colorectal cancer (CRC) remains the fourth most incident cancer in the USA with a cumulative lifetime risk of developing CRC of 5% in the general population;[15]it is the third leading cause of cancer-related deaths.[16] The relationship between body weight and different cancers is now well recognised.[17] For colon cancer, the relative risk attributable to obesity is 1.24 for men; it ranges between 1.04 and 1.13 depending on the country.[17] In 2006, there were 412 900 new cases of CRC diagnosed in 30 European countries, with an estimated personal attributable risk of 10.92% (95% CI 9.59% to 12.24%) in men and 2.57% (95% CI 0% to 5.51%) in women for colon cancer, and 5.05% (95% CI 3.45% to 6.67%) for rectal cancer in men, corresponding to 15.844 (95% CI 11.304 to 20.735) excess CRC cases.[18]
We review how obesity might promote CRC occurrence and its behaviour, and attempt to quantify the impact of both medical and surgical treatments while highlighting associated metabolic changes.
Methods
Search Strategy
We performed a systematic review searching EMBASE, MEDLINE, ISI Web of Knowledge and Pubmed using a highly sensitive search strategy to identify reports with a combination of controlled vocabulary and text words related to, first, colon or CRC (neoplasia, carcinoma, tumour, metastasis, malignancy), and second, obesity and overweight. Recursive searches and cross references were carried out using a ‘similar articles’ function and hand searches of articles identified. We included all adult human studies in French or English, published between September 1980 and May 2012.
Inclusion Criteria
We identified all meta-analyses, other systematic reviews, case–control, cohort or observational studies that assessed colon, rectal or CRC prevalence in obese compared with non-obese subjects. The WHO BMI definition of obesity ≥30 kg/m2 was adopted, but we also included studies in which BMI was defined as ≥25 kg/m2 for Asian populations.[19]
Study Selection
Data were extracted by two independent reviewers (MB and MM). When recent meta-analyses or systematic reviews were retrieved, the included studies were not reported individually. We also assessed epidemiological data that supported a relationship between obesity and CRC, such as associations with increased incidences of CRC, colorectal adenomas (CRAs), weight loss, outcomes, surgery and response to chemotherapy and/or targeted therapies.
Meta-analyses were prioritised, when available. To limit the number of references, when observational studies were too numerous to report individually, the most recent ones (past 5 years) were selected. Studies discussing pathophysiological mechanisms were assessed in a narrative way, aiming to balance the arguments for and against different biological hypotheses.
All articles addressing biological mechanisms were grouped into three subsections defined a priori: MS and visceral adipose tissue (VAT), adipocytokines, and insulin resistance and insulin-like growth factor 1 (IGF-1).
Results
From 3732 initial citations, we included 20 meta-analyses, 5 reviews, 113 observational studies and 50 additional supporting articles (figure 1). In the text and tables, results are presented as RR, IRR, HR and OR with their 95% CIs and tables.
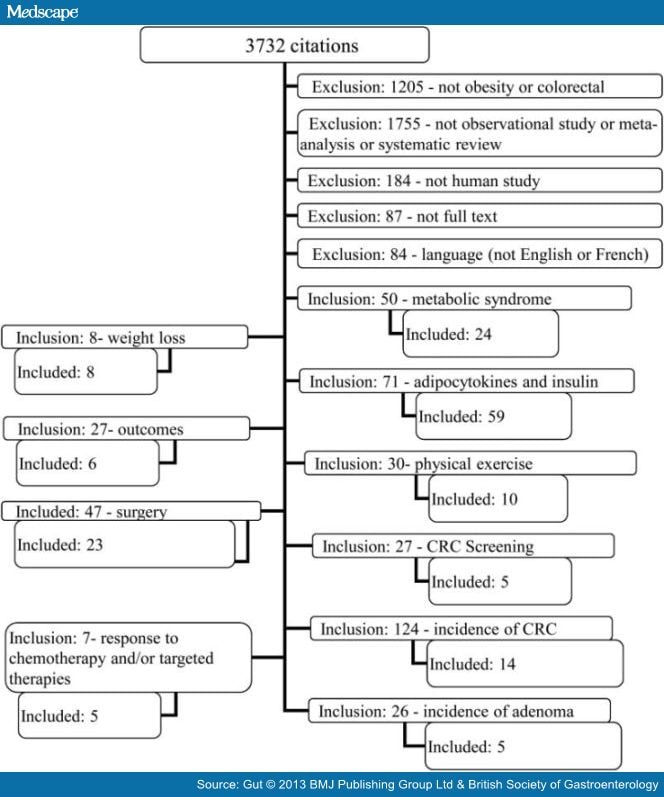
Obesity and CRC
Epidemiological Data That Support a Relationship Between Obesity and CRC
Obesity is Associated With an Increased Incidence of CRC Interpretation of results is hampered by varying methodologies and definitions of obesity. We report all published meta-analyses on this subject, and summarised the most recent studies with the largest study populations (>10 000 patients), dichotomising results by gender.
Eight meta-analyses and systematic reviews were retrieved; three were not included: Bersgstrom et al[20] included only 19 studies from 1966 to 1997; Harriss et al [21] reported on the colorectal portion of the Renehan et al [17] study (the latter was thus excluded); Ning et al [22] reported different BMI categories, making comparisons difficult. The remaining five meta-analyses published between 2007 and 2009 are detailed in Table 1 .
Men: The incidence of colon cancer, rectal cancer and CRC was significantly greater in men with obesity in all studies, with RR varying from 1.24 (1.20 to 1.28)[21] to 1.71 (1.33 to 2.19)[23] for colon cancer, from 1.09 (1.06 to 1.12)[21] to 1.75 (1.17 to 2.62)[23] for rectal cancer, and from 1.37 (1.21 to 1.56)[23] to 1.95 (1.59 to 2.39)[24] for CRC. Significant associations were also found between the incidence of cancer and men with obesity in most of the subgroups analysed, such as colon subsites, BMI and country of origin, after adjusting for physical activity, smoking, alcohol consumption or family history. Waist circumference and increasing waist-to-hip ratios were also associated with significant increases in colon cancer and CRC. The RR was not significant for rectal cancer in one study.[23]
Women: The association between obesity and colon cancer, rectal cancer or CRCs was weaker among women. Indeed, the incidence of colon cancer was found to be significantly greater in women with obesity in only one of two studies,[23,25] RR=1.12 (1.07 to 1.18), and the incidence of CRC in two of the three studies[23,24,26] RR=1.15 (1.06 to 1.24). No significant association was reported in two studies assessing rectal cancer specifically.[23,25] Subgroup analysis results varied ( Table 1 ).
Among most recent articles, Matsuo et al [27] assessed 300 000 Japanese subjects, reporting a significant association between BMI and CRC (HR (per 1 kg/m2 increase in BMI)=1.03 (1.02 to 1.04) and 1.02 (1.00 to 1.03) for men and women, respectively). Two studies showed a significant increase in colon cancer in men but not women (HR (per 5 kg/m2 increase in BMI)=1.12 (1.04 to 1.21)[28] and 1.25 (1.08 to 1.45).[29] A Chinese study including only women found a significant, U-shaped, quadratic association between BMI and colon cancer risk, with an increased risk of CRC in underweight (BMI<18.5 kg/m2) and obese subjects (p for trend=0.014), but not for rectal cancer.[30] Finally, in the last cohort study, a non-significant association was found between BMI and CRC (HR (per 1 kg/m2 increase in BMI)=1.06 (0.98 to 1.15)).[31]
In summary, BMI appears to be consistently associated with an increased risk of CRC, colon or rectal cancer in men, but less so in women. This gender difference might be explained by sex differences in prevalence and age of onset of MS, or a protective effect of oestrogen attributable to induction of apoptosis and inhibition of cell proliferation.[32]
Obesity is Associated With an Increased Incidence of CRA Four meta-analyses assessed obesity and CRA ( Table 2 ).[33–36] Although the results were expressed differently (BMI per 5-unit increase,[33]BMI cut-off value[35,36] or 10 cm increase in waist circumference[34]), all showed a small but significant association, with similar trends across races, country of origin, measured or self-reported BMI, and site other than rectal adenoma. The significance of the association persisted after adjustment for physical activity, family history of CRC, energy or alcohol intake, smoking habits or non-steroidal anti-inflammatory drug use ( Table 2 ). In addition, the most recently published meta-analysis found a dose–response relationship (Ref=BMI<25; OR=1.21 and 1.32 for BMI=25–30 and BMI=30, respectively).[36]
In contrast to CRC, increased CRA risk is observed in women and men with obesity. A meta-analysis suggested a greater but non-significant increase in CRA risk among pre-menopausal versus post-menopausal women with obesity (OR=2.48, 0.56 to 11.05; and OR=1.06, 0.77 to 1.45, respectively).[36]
A more recent case–control study comparing 1771 patients with diagnosed adenomas and 4667 polyp-free controls confirmed that a greater waist circumference was significantly associated with increased CRA risk (OR=1.32, 1.17 to 1.49).[37] In addition, MS was significantly associated with finding adenomas (OR=1.44, 1.23 to 1.70), regardless of histological type, CRA number or location other than rectum.[37]
In summary, obesity based on BMI values is consistently associated with a significant increase in CRA risk in men and women.
Weight Loss in Patients With Obesity and the Risk of CRC Few studies have assessed CRC incidence and mortality after bariatric surgery. Three studies reported on overall cancer mortality: The Swedish Obese Subjects randomised trial[38] compared 2010 patients with obesity (BMI≥34 kg/m2 in men and ≥38 kg/m2 in women) undergoing bariatric surgery with 2037 controls with obesity receiving conventional treatment,[38] while others were retrospective cohort studies.[39,40] All three studies showed that weight loss after bariatric surgery was associated with a 39–60%[38,39] risk reduction in cancer-related mortality. The numbers of deaths were too small for adequately powered site-specific analyses. Two of the studies suggested non-significant decreases in CRC incidence attributable to bariatric surgery (HR=0.52, 0.19 to 1.39 for women only;[41] and OR=0.70, 0.43 to 1.15 for men and women combined).[40]
A population-based Austrian cohort monitored changes in the weights of 28 711 men and 36 938 women for a period of 7 years, after which participants were followed for incident cancers over 8 years on average.[42] This study suggested that whereas weight change, loss or gain, had no effect on cancer incidence in all patients as a whole, weight loss (>0.10 kg/m2/year) strongly reduced the risk of CRC in men (HR=0.50, 0.29 to 0.87).[42]
In summary, the limited available data suggest weight loss may be associated with decreased CRC incidence. Weight loss obtained through diet alone or with bariatric surgery interferes with gut microbial–host metabolic crosstalk[43] and is associated with a reduction in MS,[44] and in levels of vascular endothelial growth factor (VEGF), insulin and leptin,[45] thus providing a mechanistic rationale for these clinical observations.
Obesity and Outcomes in Patients With CRC Studies assessing obesity and CRC outcomes have yielded discordant results. Meyerhardt and colleagues initially showed obesity was associated with a significant increase in overall mortality among women with stage II–III colon cancer,[46] but 5 years later in a separate study, published that BMI was not significantly associated with increased risks of colon cancer recurrence or death, although gender-specific results were not provided.[47] A cohort study of 4288 patients with Dukes B and C colon cancer showed increased recurrence or metachronous tumours (HR=1.38, 1.10 to 1.73), overall mortality (HR=1.28, 1.04 to 1.57) and colon cancer specific mortality (HR=1.36, 1.06 to 1.73) in very obese patients (BMI≥35 kg/m2).[48] More recently, the Cancer Prevention Study II Nutrition Cohort suggested that pre diagnosis, but not post diagnosis, BMI was associated with an increased risk of overall mortality (RR=1.30), colon cancer specific mortality (RR=1.35, 1.01 to 1.80) and cardiovascular mortality (RR=1.68, 1.07 to 2.65).[49]
Additional independent risk factors that have been shown to affect mortality from CRC cancer include MS (RR=2.92, 1.88 to 4.52; and RR=2.02, 0.95 to 4.27 for patients with obesity without and with MS, respectively),[50] physical exercise (PE) (improved survival in 526 CRC cases with an adjusted HR=0.73, 0.54 to 1.00) and increase in body fat (disease-specific deaths, HR (per 10 kg body fat)=1.33, 1.04 to 1.71).[51]
Other factors explaining the increased risk of mortality probably include late diagnosis, aggressiveness of the cancer and diminished treatment response.
In summary, it is suggested, although inconsistently, that obesity might be associated with a decrease in overall survival in patients with CRC, independently of MS, with possible gender-specific differences.
Factors That Confound the Association Between Obesity and CRC
Physical Exercise (PE) Two recent meta-analyses, by the same authors, found that PE was associated with a significantly decreased risk of CRC (OR=0.76, 0.71 to 0.82)[52] and CRA (OR=0.84, 0.77 to 0.92).[53] It has been speculated that obesity may be associated with specific eating behaviours, such as high consumption of red or processed meat or low consumption of fibres or folate, favouring CRC occurrence. Several studies, however, have found that the protective effects of PE and the deleterious effects of obesity persisted after adjustment for these possible confounding factors.[54,55] A recent systematic review suggested PE was associated with a decrease in most of the factors discussed below, such as insulin, insulin resistance, IGF-1, IGF binding protein 3 (IGFBP-3) or leptin.[56] Physical activity can also decrease colon transit time, particularly in the recto-sigmoid region, and thus contact time of alimentary carcinogens with the colon mucosa.[57] However, previous reports have suggested physical activity does not necessarily improve gastrointestinal transit.[58]
Another link between obesity, PE and CRC might be related to low levels of vitamin D, which have been associated with an increase in CRC risk, irrespective of BMI.[59] Patients with obesity may exhibit low levels of vitamin D for at least two reasons: the sequestration of vitamin D in adipose tissue[60] and a decrease in production.
CRC Screening The increased prevalence of CRC in patients with obesity might be partly explained by reduced adherence to screening strategies. A study comparing screening of normal weight patients to that of the latter displayed a reduced rate of CRC screening, whether by opportunistic colonoscopy or faecal occult blood testing.[61] A recently published meta-analysis highlighted possible racial disparities, suggesting that BMI was not associated with CRC screening.[62] Nevertheless, white women and men with obesity had lower rates of CRC screening than their normal BMI counterparts. For women, the trend was significantly associated with obesity classes.[62] Additional findings were reported in a prospective study that assessed reporting of CRC screening and the probability of agreeing with statements denoting attitudes/perceptions about CRC and screening.[63] This study showed that overweight women and those with obesity were 40% less likely to undergo CRC screening compared with normal-weight subjects. The reason for this lower adherence to screening policy in subjects with obesity has yet to be determined. Indeed, the study by Messina et al [63] suggested that although women with obesity were less aware than those with normal BMI that obesity increased the risk of CRC (OR=0.5, 0.3 to 0.9), and were less worried about CRC (OR=0.5, 0.3 to 0.8), these factors were unlikely to explain the observed differences in screening rates.
In summary, decreased adherence to CRC screening policies, more pronounced in women than men, does not explain the observed association between obesity and increased risks of CRC in men, or CRA in men and women. However, decreased screening adherence may be associated with the weaker association observed between obesity and CRC in women. There are several limitations in the data supporting the impact of decreased adherence to screening policies among subjects with obesity and the increased risk of CRC. First, the absolute difference is quite small, usually less than 10%;[61,63]second, the findings were mostly described for women with morbid obesity.[61–63] It therefore seems unlikely that reduced cancer screening in subjects with obesity would be sufficient to account for any significant differences.
Relationships Between Obesity, Treatments and CRC Outcomes
Obesity and Surgery for CRC Our systematic review did not identify any existing meta-analyses or systematic reviews that assessed short-term surgical outcomes for CRC in patients with obesity.
A meta-analysis of eight studies[64] and one narrative review that included 33 studies[65] reported on laparoscopic colorectal surgery among patients with obesity. Both reviews concluded obesity was associated with increased conversion rates, operating times and postoperative morbidity, whereas no significant impact of obesity was evidenced on other outcomes such as intraoperative blood loss, the number of dissected lymph nodes, perioperative mortality and reoperation rates.
Twenty fully published observational studies were retrieved during the literature search for CRC surgery in patients with obesity.[66–85] Of these, 12 included the use of laparoscopic operative techniques alone,[66,68,70,74–76,79,81–84,86] while 7 were Asian studies defining obesity as BMI≥25 kg/m2 [66,74,75,79,84–86] or ≥28 kg/m2.[82] Duration of hospital stay was the most commonly reported outcome, with only 3 of 16 studies reporting significantly longer stays for patients with obesity.[66,71,86]
Three of 16 studies reported significantly increased complication rates in patients with obesity,[66,78,83]whereas none reported increased mortality. All four studies reporting on intraoperative blood loss showed significantly greater values in patients with obesity.[66,67,81,86] Three of nine studies found significantly more wound infection in patients with obesity.[66,68,83] Sepsis was assessed in three studies, with no significant differences.[67,73,78]
A significantly greater number of nodes were removed in patients without obesity in 1 of 12 studies.[66]Park et al [86] reported a larger number of lymph nodes removed in patients without obesity than in patients with BMI=25.0–29.9 kg/m2, but no differences compared with patients with higher BMI (>30.0 kg/m2).
Overall, no clear relationship has been shown between obesity and operative and postoperative outcomes except for a modest but significant trend in overall complication rates. Meaningful contemporary summary data are needed.
Obesity and Response to Chemotherapy and/or Targeted Therapies It has been suggested that obesity, particularly visceral obesity, and its related metabolic changes promote angiogenesis.[87] Thus it can be speculated that patients with obesity might exhibit an impaired response to chemotherapy, particularly protocols that include targeted therapies. In addition, in some patients with obesity, increased plasma levels of insulin may further decrease therapeutic effect.[88] A study of 120 patients with metastatic CRC receiving bevacizumab-based treatment (n=80) or chemotherapy alone (n=40)[89]found that high BMI, and visceral and subcutaneous fat areas were significantly associated with absence of response to the bevacizumab-based treatment but not the chemotherapy group.[89] Mean time to progression (TTP) was significantly shorter in patients with high BMI values (9 vs 12 months; p=0.01). In a multivariable analysis, high visceral fat area was independently associated with response, TTP and overall survival (HR=7.18, 1.69 to 30.6; HR=2.80, 1.35 to 5.79; and HR=2.88, 1.13 to 7.32, respectively).[89] These results were confirmed by others in a separate cohort of 49 consecutive patients (VFA significantly lower in respondents 111.9±12 cm2 vs non-respondents 210.8±58 cm2, p=0.03).[90]Indirect support for these findings was also provided by a recent analysis of two large phase III studies (the CAIRO and CAIRO2 studies)[91] in which a high BMI was associated with better median overall survival for patients receiving chemotherapy alone versus targeted therapy plus chemotherapy. The authors hypothesised a possible decreased efficacy of bevacizumab in patients with obesity.
The Rationale for the Reported Relationships Between Obesity and CRC
A quick overview is presented in figure 2.
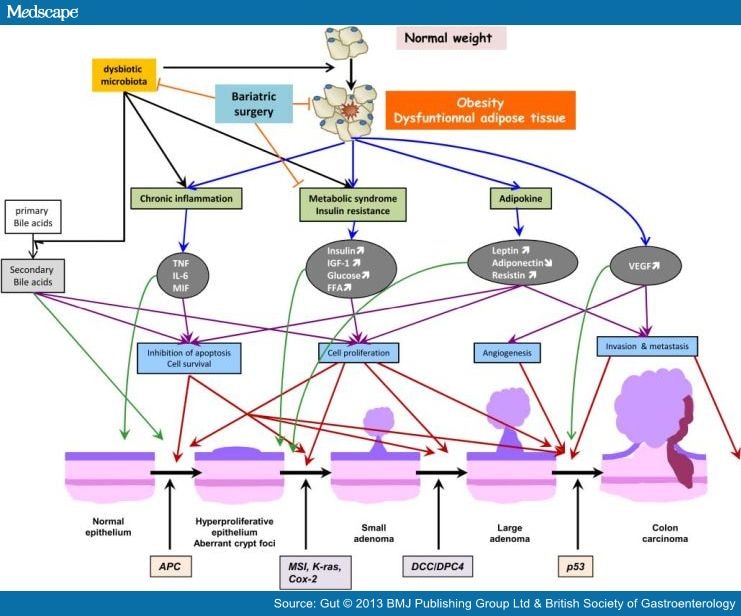
Figure 2. Summary of potential factors that are believed to relate obesity and colorectal cancer. Blue arrows indicate the metabolic consequences of obesity. Black arrows are for some of the suspected consequences of dysbiotic microbiota. Purple arrows are for the cellular events induced by obesity-related metabolic changes. Red arrows locate these cellular events in the carcinogenic process. Green arrows suggest the stage of the normal epithelium-to-carcinoma sequence when the different biological factors might start to act. And finally, orange lines suggest some of the potentially beneficial effects of bariatric surgery. FFA, free fatty acid; IGF-1, insulin-like growth factor 1; IL, interleukin; MIF, macrophage migration inhibitory factor; TNF, tumour necrosis factor; VEGF, vascular endothelial growth factor. APC, Adenomatous polyposis coli; MSI, microsatelite instability, K-ras, Kirsten-rat sarcoma, Cox-2, cyclooxygenase-2; DCC (deleted in colorectal carcinomas), DPC4 (deleted in pancreatic carcinomas, locus 4).
MS and Visceral Adipose Tissue The term MS describes a state of metabolic dysregulation characterised by insulin resistance with increased fasting glucose, hyperinsulinaemia, proinflammatory and procoagulant changes, and a predisposition to type 2 diabetes, dyslipidaemia (increased triglycerides and reduced high-density lipoprotein cholesterol), premature atherosclerosis and other disorders.[92]
The occurrence of MS is strongly affected by the presence of visceral obesity, or VAT, and is influenced by hormonal factors,[7,93] but obesity is a remarkably heterogeneous condition and not every patient with obesity is characterised by comorbidities and the presence of MS.[94] In this regard, body fat distribution, especially VAT accumulation, has been found to be a major correlate of the MS cluster.[7]
Metabolic Syndrome In the metabolic syndrome and cancer project (Me-Can), a prospective international population-based study of 580 000 people assessing MS and cancer risk, after 12 years of follow-up, 2834 men and 1861 women had been diagnosed with CRC (RR=1.25 for men, 1.18 to 1.32; and 1.14 for women, 1.02 to 1.18).[95] Two studies reported a risk increase for CRC (OR=1.09, 0.74 to 1.60; OR=2.40, 1.36 to 4.25; and OR=2.57, 1.20 to 5.52 for one, two and three components vs none, respectively)[96] and CRA (OR=1.14, 1.38, 1.61*, 2.57* and 3.23* for one, two, three, four and five components vs none, respectively, *p<0.05)[97] as the number of MS components rises. Some authors suggested that MS, and more specifically waist circumference, appears to be an independent risk factor for CRA,[97,98] whereas others have found no such relationship.[99]
It has been suggested that there is an interaction between hormonal factors and MS, as the risk of CRC with MS was either show to be observed only in men (OR=1.86, 1.21 to 2.86; OR=1.13, 0.66 to 1.93 for men and women, respectively)[100] or to be stronger in men (RR for CRC= 1.78 and 1.16 for men and women, respectively).[101] The same findings were reported for CRA (OR=1.44, 1.16 to 1.80; OR=1.04, 0.74 to 1.46 for men and women, respectively).[102]
However, the relationship with gender appears complex as MS is associated with an increased likelihood of metachronous neoplasia (mainly adenomas) among women (OR=1.37, 1.01 to 1.85) but not men (OR=0.99, 0.81 to 1.21).[103] However, when the analysis was restricted to advanced adenoma, waist circumference was associated with a significant risk in men (OR=1.41, 1.05 to 1.90) but not in women (OR=0.89, 0.55 to 1.42).[103] A small study suggested that worsened CRC outcomes in men with MS might be explained by a more aggressive phenotype.[104]
The other components of MS, that is, dyslipidaemia, hypertension and insulin resistance, also independently increase CRC[105] and CRA risks.[37]
A study that included 23 962 patients without MS and 9268 with MS (visceral obesity 7.2% and 55.9%, respectively) followed for 14.4 years found that MS was significant increased in those with CRC mortality (HR=2.15, 1.27 to 3.62).[50] Statistical significance was lost when analysis was restricted to colon cancer mortality (HR=1.72, 0.97 to 3.08).[50]
To summarise, there appears to be an association between MS and both CRA and CRC incidence and prognosis. These associations are stronger in men, yet their magnitude and the presence of possible confounding deserve further characterisation.
Several biological factors may explain the role of MS in the development and outcome of CRC: the most important is insulin resistance, while other factors include the associated increased production of insulin (IGF-1) and endothelial growth factors, and varying levels of several adipocytokines.[106]
Visceral Adipose Tissue VAT has been identified as a risk factor for CRC (magnitude of risk ranging from RR=1.9, 1.1 to 3.3[107] to 4.01, 1.0 to 16.4[108]) and CRA (RR=1.58, 1.11 to 2.24),[109] either independently or in relation with other factors such as adiponectin (APN).[110] VAT volume is associated with CRA prevalence independently of BMI, with the strength of association varying across studies (example of ranges: from RR=1.6, 1.10 to 2.20,[109] to RR=5.92, 9% CI 1.22 to 28.65[111]). VAT may be a more accurate marker than waist circumference for increased CRC risk;[112] nevertheless the association between VAT and CRC has been questioned.[113]
Adipocytokines Adipose tissue produces various growth factors, hormones and cytokines, known as adipocytokines. The adipocytokines include leptin, resistin, visfatin, Adiponectin (APN) and numerous cytokines including tumour necrosis factor α, interleukin (IL)-6, IL-8, IL-10 and IL-1 receptor agonist. Experimental and epidemiological data have shown that obesity leads to altered levels of several adipocytokines,[114] further contributing to an increased risk of CRC.
Adiponectin APN is an adipocyte-derived insulin-sensitising hormone with circulating levels inversely proportional to obesity.[115] Following binding to two main receptors, AdipoR1 and AdipoR2, APN activates several intracellular signalling pathways (mainly adenosine monophosphate-activated protein kinase (AMPK) but also mammalian target of rapamycin (mTOR) and others), with resulting signal transduction and activator of transcription.[116]
A recent meta-analysis of 13 studies suggested that APN had a protective effect against CRC as, in men only, a 1 μg/ml increase in APN levels was associated with a 2% decrease in the risk of colorectal neoplasia.[117] This inverse association was confirmed by a second meta-analysis, which included 2632 cases of CRC or adenoma and 2753 healthy controls,[118] and a recent case–control study not included in the two meta-analyses.[119] It was suggested that low plasma levels of APN might be associated with CRC risk independently of other anthropometric measurements and other potential confounders, including family history, physical activity, current smoking and aspirin use.[120] Once again, the association between plasma levels of APN and the risk of CRC or CRA is controversial as some groups have disparate results.[121–123]
Plasma levels of APN and AdipoR1 and AdipoR2 expression in CRC tissue have been inversely associated with tumour differentiation.[124,125] In addition, low presurgical APN levels are found more frequently in patients with relapsing disease compared with non-relapsing disease (52% vs 26% respectively).[124] Treatment-induced variations in APN levels may also predict local recurrences and/or distant metastases in patients with rectal cancer treated with cetuximab.[126]
Several actions of APN could be involved in this protective effect against CRC. Though controversial,[127] APN has been described as exerting antiangiogenic properties leading to a significant inhibition of liver tumour growth and metastasis,[128] and CRC cell growth.[129] Other possible explanatory mechanisms for the anticancer role of APN include inhibition of cell proliferation,[130] and the induction of macrophage infiltration in tumours[131] and apoptosis.[130] APN may also suppress colon epithelial cell proliferation via inhibition of the mTOR pathway after a high-fat but not a standard diet.[132] Indeed, the number of chemically induced colon polyps was significantly greater in APN-deficient mice than in wild-type mice fed a high-fat diet (HFD), but not in APN-deficient mice fed a standard diet.[132] Finally, experimental studies have suggested that APN may play an important role in CRC prevention by modulating genes involved in chronic inflammation and tumourigenesis.[133]
To summarise, decreased levels of APN appear to be associated with an increased risk of CRC, although there are some discrepant studies. In addition, APN levels and AdipoR1 and AdipoR2 tumour expression might bear prognostic value.
Leptin Unlike APN, leptin is mainly secreted by white adipose tissue, and circulating levels of leptin are higher in patients with obesity than in those without. The level of expression correlates with the severity of the obesity.[134,135] The binding of leptin to its receptors, ObRb, which are expressed in the colon epithelium, activates several signal transduction pathways, including Janus kinase signal transducer and activator of transcription 3, phosphatidylinositol 3 kinase (PI3K), and other pathways, such as the mitogen-activated protein kinase (MAPK), 5’AMPK, and the mTOR pathways, which have been implicated in CRC.[136,137]
Although some studies reported normal serum leptin levels in patients with CRC,[138,139] a few case–control studies have shown an elevated risk of CRC associated with high serum leptin levels.[140–142] A case–control study nested within the Women’s Health Initiative cohort of postmenopausal women, found that after adjustment for age, race, smoking, colonoscopy history, oestrogen level and insulin, only leptin remained significantly associated with CRC risk (HR comparing quartile four to one=1.84, 1.17 to 2.90).[143]
In addition to increasing CRC risk, plasma leptin and leptin receptor levels have been associated with more aggressive tumour phenotype.[104,144] An additional study of 108 Chinese patients with CRC reported a significant association between leptin/Ob-R expression and T and TNM staging, lymph node metastasis, distant metastasis, differentiation, and increased expression of phosphorylated PI3K, Akt and mTOR protein, suggesting that leptin may regulate the proliferation and apoptosis of CRC through the PI3K/Akt/mTOR signalling pathway.[145] However, a small study of 37 patients with CRA, 36 with CRC, and 25 controls found significantly lower plasma leptin levels in patients with CRC compared with those with CRA or controls.[146] Several factors might explain these discrepancies, including the realisation that leptin is not measured using a routine, standardised method, leading to large across-study differences.[104,143,144, 146]
Studies assessing Ob-R tissue sample expression in CRC and normal adjacent mucosa found greater Ob-R expression in about 80% of CRC specimens; Ob-R expression also correlated with several clinicopathological parameters such as tumour differentiation and the presence of metastases.[135,147,148] Surprisingly both studies suggested that among patients with tumour, those with reduced Ob-R expression had worse outcomes than those with Ob-R overexpression, expressed either as progression free[135] or overall survival.[148] The study by Uddin et al [148] is limited by its retrospective design, a 16-year span during which CRC management evolved, and the subjective quantification of Ob-R expression.
Numerous studies have reported the carcinogenic effects of leptin, but overall, the role of leptin in CRC induction and growth remains unclear. Leptin inhibits apoptosis in human colon cancer cell lines and thus promotes proliferation of normal colonic epithelial and cancer cells.[148–150] A recent study suggested that leptin acts as a growth factor for CRC.[149] However, leptin has been shown by others to reduce the development of chemically induced precancerous colonic lesions—a finding that contradicts a possible role for leptin in fostering carcinogenesis.[151] These findings suggest that the relationship between obesity and CRC is multifactorial.[152] The discrepant observations may be explained by differing effects of leptin in in vitro and in vivo experiments and among different cell types; the genetic background may also be a factor, notably the Apc genotype, as detailed elsewhere.[153]
The role of leptin receptor (ObRb) polymorphism has also been investigated, but with no demonstrated association with CRA risk.[154] However, this study confirmed an increase, 3.3 fold, in CRA for men exhibiting the highest leptin concentrations.
In summary, epidemiological studies support, although not unanimously, a relationship between high plasma levels of leptin and CRC risk. Experimental data suggest leptin may be involved in tumour growth rather than initiation.
Insulin Resistance and IGF-1 Obesity is associated with an increase in insulin release and a decrease in insulin sensitivity, mediated by the decreased expression of insulin-receptor levels and reduced intracellular insulin signalling in response to insulin receptor binding.[155] This results in hyperinsulinaemia and insulin resistance,[156] with an associated increase in IGF levels. IGF-1 and IGF-2 are bound by six high-affinity binding proteins (IGFBP1–6) and other low-affinity binding proteins (IGFBP-related proteins); the higher the plasma levels of insulin, the greater the bioavailability of IGF through reduction of IGFBP1 and 2.[157] IGF is involved in the control of normal growth, maintenance of tissue homeostasis and a differentiated phenotype, alterations in the balance of proliferation and apoptosis, angiogenesis, cell adhesion, migration and wound healing.[158]
There is strong evidence from animal and human studies that cancer development is promoted by high concentrations of insulin and IGFs acting through the insulin/IGF axis. Stimulation of the IGF-1 receptor (IGF-1R), a tyrosine kinase, activates two main signalling pathways, PI3K-AKT and RAS-Raf-MAPK, which have multiple effects on gene regulation and protein expression, activation and translocation.[159,160] Another important pathway, the c-Jun N-terminal kinase (JNK) pathway, one of the subfamilies of MAPK, appears to play a crucial role in obesity and insulin resistance, and in colorectal carcinogenesis. Experimental data have shown that a HFD might increase insulin levels, inactivate AKT and increase JNK activity.[161] Interestingly, in this study, a HFD was associated with an increase in the number of aberrant crypt foci and the proliferation of colon epithelial cells. Both these effects were prevented by the use of a JNK inhibitor.[161] The IGF-1 receptor (IGF1-R) is expressed in normal non-transformed colon mucosa, and in human CRCs.[162] However, silencing or knocking out IGF-1R inhibits cell proliferation and enhances the sensitivity of human colon cancer cells to chemotherapy.[163] The deregulation of this pathway can thus give rise to malignancy.
Epidemiological studies and meta-analyses have assessed the relationship between IGF and CRC or CRA, but not specifically in patients with obesity. A recent 11-study meta-analysis, incorporating the most up-to-date information, reported a non-significant association between IGF-1 and CRC risk (RR=1.13, 0.97 to 1.32),[164] raising doubts about the findings of an older meta-analysis that had suggested a significant association between IGF-1 and increased CRC risk (OR=1.58, 1.11 to 2.27) based on fewer studies.[165]
As for a possible association between IGF and CRA, levels of IGF-I and insulin, and the IGF-I/IGFBP-3 ratio have been shown to be significantly greater in subjects with CRA compared with controls (OR 1.7, 1.0 to 2.9).[166] This finding was confirmed in a more recent study of 143 subjects, nested in a larger cohort study,[167] that showed subjects experiencing an increase of at least 50% in either IGF-1 or IGFBP3 exhibited a significantly greater CRA risk (OR for ever increase vs no increase=3.81, 1.30–10.8; and 2.83, 1.00–8.22 for IGF-1 and IGF-1/IGFBP3, respectively).[167]
In support of a gender difference in the relationship between obesity and CRC or CRA, a study conducted in healthy and lean subjects undergoing colonoscopy found a positive association between IGF-I and CRA (OR=1.63, 1.08 to 2.46), and an inverse correlation between IGFBP-1 and CRA (OR=0.49, 0.32 to 0.75) in men; no significant associations were found in women, suggesting insulin levels and the IGF axis act differently on colorectal carcinogenesis among men and women, at least at an early stage.[168]
A recently published case–control study that included African-American (231 cases and 306 controls) and white (297 cases, 530 controls) patients assessed the relationships between IGF-1 (CA)19 , IGF-2Apa1, IGFBP-3, and APN gene polymorphism and CRC risk.[169] In white patients only, those homozygous for the IGF-1 (CA)19 repeat exhibited an increased CRC risk (OR=1.77, 1.15 to 2.73), while those carrying the IGF-2 Apa1 A-variant exhibited a decreased CRC risk (OR=0.49, 0.28 to 0.88).[169] The investigators also suggested specific variants of IGF-2R might be associated with increased CRC risk (OR=2.2, 0.9 to 5.4), perhaps explained by modulating circulating levels of IGF-2.[170]
In keeping with clinical observations that suggest a more severe course of CRC in patients with obesity, it has also been suggested that IGF-I receptor might be implicated in the promotion of liver metastases.[171]
To summarise, insulin resistance and IGF-1 are associated with a slight increase in CRC and greater increase in CRA risks. As supportive data are few, even though there exists biological plausibility for a link between insulin resistance, IGF-1 and colorectal tumours, more epidemiological data are needed.
Other Biological Factors That May Support a Link Between Obesity and CRC: Inflammation, Bile Acids and the Microbiota We have so far considered the putative explanatory roles of several biological entities, but numerous additional factors may be involved, such as inflammation, bile acids and the microbiota, that is, the human gut flora. Some of these, such as inflammation and bile acids, have been well studied, whereas the microbiota represents an emerging field of interest.[172] As most of these factors are inter related, any relationships with CRC are likely complex. Indeed, obesity and MS have long been recognised as chronic subclinical inflammatory conditions that may underlie increased CRC risk.[173] Several mechanisms can link inflammation to CRC, including oxidative stress, which in turn can affect the regulation of genes encoding for factors that play a role in colorectal carcinogenesis, such as p53, DNA mismatch repair proteins, and base-excision DNA-repair proteins, to name a few.[174]It has been suggested that microbiota is involved in the development of low-grade inflammation associated with obesity.[175] However, specific bacterial strains of the microbiota can exert anti-inflammatory properties.[176] In addition, the gut microbiota are well recognised as a predisposing factor for obesity, and are now considered one of the most important environmental factors with an impact on host physiology and metabolism.[177]
Bile acids are produced in the liver by the metabolism of cholesterol and are composed of cholic acid and chenodeoxycholic acid. These primary bile acids are converted to secondary bile acids, mainly deoxycholic acid and lithocholic acid, by the colonic microbiota. These secondary bile acids affect cancer development through tumour-promoting activities, and by inducing DNA damage and apoptosis.[178] It has also been suggested, in animal models, that bile acids can regulate the composition of the gut microbiota.[179] Although the microbiota is essential and beneficial to the host, various events, such as infection, diet, stress or inflammation, may impact microbial composition, leading to the formation of dysbiotic microbiota. It is now suggested that there is a direct relationship between the gut microbiota and the development of CRC.[180] To summarise, bile acids, inflammation and the gut microbiota have all been related to CRC; obesity and the microbiota can induce inflammation; obesity and a HFD can alter the microbiota and, conversely, the microbiota can promote obesity; and secondary bile acids are produced by the gut microbiota and can in turn alter microbiota composition and favour the development of tumours.
The involvement of bile acids in CRC might explain some of the aforementioned gender differences as hepatic bile acid synthesis is 15% lower in women taking oestrogen, thereby reducing this stimulus.[181,182]
Additional adipocytokines that may be of interest include resistin, a member of the newly discovered family of cysteine-rich proteins called ‘resistin-like molecules’.[183]
Conclusions
This up-to-date review highlights some of the most important and recent findings characterising the relationship between obesity and CRC, while identifying possible underlying mechanisms, most of which remain controversial. Obesity increases the prevalence of CRC and influences outcomes, overall and in relation to specific CRC treatments, especially VEGF-targeting therapies. Weight loss, mostly after gastric bypass surgery, bears a significant impact on the course of CRC. A number of questions remain unanswered. How do we optimise the use of possible biological and clinical predictors such as leptin, APN, VAT area and the MS? How do we better assess CRC in patients with obesity, adapt CRC screening policies, and perhaps improve indications for gastric bypass surgery?
References
- Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012;307:491–7.
- Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 2012;307:483–90.
- Bogossian FE, Hepworth J, Leong GM, et al. A cross-sectional analysis of patterns of obesity in a cohort of working nurses and midwives in Australia, New Zealand, and the United Kingdom. Int J Nurs Stud 2012;49:727–38.
- Charles MA, Eschwege E, Basdevant A. Monitoring the obesity epidemic in France: the Obepi surveys 1997–2006.Obesity (Silver Spring) 2008;16:2182–6.
- Xi B, Liang Y, He T, et al. Secular trends in the prevalence of general and abdominal obesity among Chinese adults, 1993–2009. Obes Rev 2012;13:287–96.
- Abajo A, Bitarte N, Zarate R, et al. Identification of colorectal cancer metastasis markers by an angiogenesis-related cytokine-antibody array. World J Gastroenterol 2012;18:637–45.
- Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881–7.
- Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med 2008;168:1617–24.
- Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med 2008;168:1609–16.
- Golan R, Shelef I, Rudich A, et al. Abdominal superficial subcutaneous fat: a putative distinct protective fat subdepot in type 2 diabetes. Diabetes Care 2012;35:640–7.
- Evans PD, McIntyre NJ, Fluck RJ, et al. Anthropomorphic measurements that include central fat distribution are more closely related with key risk factors than BMI in CKD stage 3. PLoS One 2012;7:e34699.
- Lovejoy JC, de la Bretonne JA, Klemperer M, et al. Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism 1996;45:1119–24.
- Albu JB, Murphy L, Frager DHJ, et al. Visceral fat and race-dependent health risks in obese nondiabetic premenopausal women. Diabetes 1997;46:456–62.
- Carroll JF, Fulda KG, Chiapa AL, et al. Impact of race/ethnicity on the relationship between visceral fat and inflammatory biomarkers. Obesity (Silver Spring) 2009;17:1420–7.
- Andrieu N, Launoy G, Guillois R, et al. Familial relative risk of colorectal cancer: a population-based study. Eur J Cancer 2003;39:1904–11.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10–29.
- Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–78.
- Renehan AG, Soerjomataram I, Tyson M, et al. Incident cancer burden attributable to excess body mass index in 30 European countries. Int J Cancer 2010;126:692–702.
- Examination Committee of Criteria for ‘Obesity Disease’ in Japan; Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circ J 2002;66:987–92.
- Bergstrom A, Pisani P, Tenet V, et al. Overweight as an avoidable cause of cancer in Europe. Int J Cancer2001;91:421–30.
- Harriss DJ, Atkinson G, George K, et al. Lifestyle factors and colorectal cancer risk (1): systematic review and meta-analysis of associations with body mass index. Colorectal Dis 2009;11:547–63.
- Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev 2010;11:19–30.
- Dai Z, Xu YC, Niu L. Obesity and colorectal cancer risk: a meta-analysis of cohort studies. World J Gastroenterol2007;13:4199–206.
- Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. Bmc Public Health 2009;9:88.
- Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr 2007;86:556–65.
- Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev 2007;16:2533–47.
- Matsuo K, Mizoue T, Tanaka K, et al. Association between body mass index and the colorectal cancer risk in Japan: pooled analysis of population-based cohort studies in Japan. Ann Oncol 2012;23:479–90.
- Bassett JK, Severi G, English DR, et al. Body size, weight change, and risk of colon cancer. Cancer Epidem Biomar 2010;19:2978–86.
- Laake I, Thune I, Selmer R, et al. A prospective study of body mass index, weight change, and risk of cancer in the proximal and distal colon. Cancer Epidemiol Biomarkers Prev 2010;19:1511–22.
- Odegaard AO, Koh WP, Yu MC, et al. Body mass index and risk of colorectal cancer in Chinese Singaporeans: the Singapore Chinese Health Study. Cancer 2011;117:3841–9.
- Burton A, Martin R, Galobardes B, et al. Young adulthood body mass index and risk of cancer in later adulthood: historical cohort study. Cancer Causes Control 2010;21:2069–77.
- Chen J, Iverson D. Estrogen in obesity-associated colon cancer: friend or foe? Protecting postmenopausal women but promoting late-stage colon cancer. Cancer Causes Control 2012;23:1767–73.
- Ben Q, An W, Jiang Y, et al. Body mass index increases risk for colorectal adenomas based on meta-analysis.Gastroenterology 2012;142:762–72.
- Hong S, Cai Q, Chen D, et al. Abdominal obesity and the risk of colorectal adenoma: a meta-analysis of observational studies. Eur J Cancer Prev 2012;21:523–31.
- Myung SK, Lee YJ, Cho B, et al. Adiposity and the risk of colorectal adenomatous polyps: a meta-analysis. Cancer Cause Control 2011;22:1021–35.
- Okabayashi K, Ashrafian H, Hasegawa H, et al. Body mass index category as a risk factor for colorectal adenomas: a systematic review and meta-analysis. Am J Gastroenterol 2012;107:1175–85; quiz 86.
- Kim BC, Shin A, Hong CW, et al. Association of colorectal adenoma with components of metabolic syndrome.Cancer Causes Control 2012;23:727–35.
- Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–52.
- Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med2007;357:753–61.
- Adams TD, Stroup AM, Gress RE, et al. Cancer incidence and mortality after gastric bypass surgery. Obesity (Silver Spring) 2009;17:796–802.
- Sjostrom L, Gummesson A, Sjostrom CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol 2009;10:653–62.
- Rapp K, Klenk J, Ulmer H, et al. Weight change and cancer risk in a cohort of more than 65,000 adults in Austria.Ann Oncol 2008;19:641–8.
- Li JV, Ashrafian H, Bueter M, et al. Metabolic surgery profoundly influences gut microbial–host metabolic cross-talk. Gut 2011;60:1214–23.
- Nugent C, Bai C, Elariny H, et al. Metabolic syndrome after laparoscopic bariatric surgery. Obes Surg2008;18:1278–86.
- Garcia de la Torre N, Rubio MA, Bordiu E, et al. Effects of weight loss after bariatric surgery for morbid obesity on vascular endothelial growth factor-A, adipocytokines, and insulin. J Clin Endocrinol Metab 2008;93:4276–81.
- Meyerhardt JA, Catalano PJ, Haller DG, et al. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer 2003;98:484–95.
- Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from Cancer and Leukemia Group B 89803. J Clin Oncol 2008;26:4109–15.
- Dignam JJ, Polite BN, Yothers G, et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst 2006;98:1647–54.
- Campbell PT, Newton CC, Dehal AN, et al. Impact of body mass index on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol 2012;30:42–52.
- Matthews CE, Sui X, LaMonte MJ, et al. Metabolic syndrome and risk of death from cancers of the digestive system. Metabolism 2010;59:1231–9.
- Haydon AM, Macinnis RJ, English DR, et al. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut 2006;55:62–7.
- Wolin KY, Yan Y, Colditz GA, et al. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer2009;100:611–6.
- Wolin KY, Yan Y, Colditz GA. Physical activity and risk of colon adenoma: a meta-analysis. Br J Cancer2011;104:882–5.
- Giovannucci E, Ascherio A, Rimm EB, et al. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med 1995;122:327–34.
- Guilera M, Connelly-Frost A, Keku TO, et al. Does physical activity modify the association between body mass index and colorectal adenomas? Nutr Cancer 2005;51:140–5.
- Winzer BM, Whiteman DC, Reeves MM, et al. Physical activity and cancer prevention: a systematic review of clinical trials. Cancer Causes Control 2011;22:811–26.
- Song BK, Cho KO, Jo Y, et al. Colon transit time according to physical activity level in adults. J Neurogastroenterol Motil 2012;18:64–9.
- Robertson G, Meshkinpour H, Vandenberg K, et al. Effects of exercise on total and segmental colon transit. J Clin Gastroenterol 1993;16:300–3.
- Neuhouser ML, Manson JE, Millen A, et al. The influence of health and lifestyle characteristics on the relation of serum 25-hydroxyvitamin d with risk of colorectal and breast cancer in postmenopausal women. Am J Epidemiol2012;175:673–84.
- Earthman CP, Beckman LM, Masodkar K, et al. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int J Obes (Lond) 2012;36:387–96.
- Rosen AB, Schneider EC. Colorectal cancer screening disparities related to obesity and gender. J Gen Intern Med2004;19:332–8.
- Maruthur NM, Bolen S, Gudzune K, et al. Body mass index and colon cancer screening: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2012;21:737–46.
- Messina CR, Lane DS, Anderson JC. Body mass index and screening for colorectal cancer: gender and attitudinal factors. Cancer Epidemiol 2012;36:400–8.
- Zhou Y, Wu L, Li X, et al. Outcome of laparoscopic colorectal surgery in obese and nonobese patients: a meta-analysis. Surg Endosc 2012;26:783–9.
- Makino T, Shukla PJ, Rubino F, et al. The impact of obesity on perioperative outcomes after laparoscopic colorectal resection. Ann Surg 2012;255:228–36.
- Akiyoshi T, Ueno M, Fukunaga Y, et al. Effect of body mass index on short-term outcomes of patients undergoing laparoscopic resection for colorectal cancer: a single institution experience in Japan. Surg Laparosc Endosc Percutan Tech 2011;21:409–14.
- Ballian N, Yamane B, Leverson G, et al. Body mass index does not affect postoperative morbidity and oncologic outcomes of total mesorectal excision for rectal adenocarcinoma. Ann Surg Oncol 2010;17:1606–13.
- Bege T, Lelong B, Francon D, et al. Impact of obesity on short-term results of laparoscopic rectal cancer resection.Surg Endosc 2009;23:1460–4.
- Blee TH, Belzer GE, Lambert PJ. Obesity: is there an increase in perioperative complications in those undergoing elective colon and rectal resection for carcinoma? Am Surg 2002;68:163–6.
- Blumberg D. Laparoscopic colectomy performed using a completely intracorporeal technique is associated with similar outcome in obese and thin patients. Surg Laparosc Endosc Percutan Tech 2009;19:57–61.
- Chern H, Chou J, Donkor C, et al. Effects of obesity in rectal cancer surgery. J Am Coll Surg 2010;211:55–60.
- Damadi AA, Julien L, Arrangoiz R, et al. Does obesity influence lymph node harvest among patients undergoing colectomy for colon cancer? Am Surg 2008;74:1073–7.
- Healy LA, Ryan AM, Sutton E, et al. Impact of obesity on surgical and oncological outcomes in the management of colorectal cancer. Int J Colorectal Dis 2010;25:1293–9.
- Ishii Y, Hasegawa H, Nishibori H, et al. Impact of visceral obesity on surgical outcome after laparoscopic surgery for rectal cancer. Br J Surg 2005;92:1261–2.
- Kang J, Baek SE, Kim T, et al. Impact of fat obesity on laparoscopic total mesorectal excision: more reliable indicator than body mass index. Int J Colorectal Dis 2012;27:497–505.
- Karahasanoglu T, Hamzaoglu I, Baca B, et al. Impact of increased body mass index on laparoscopic surgery for rectal cancer. Eur Surg Res 2011;46:87–93.
- Linebarger JH, Mathiason MA, Kallies KJ, et al. Does obesity impact lymph node retrieval in colon cancer surgery?Am J Surg 2010;200:478–82.
- Merkow RP, Bilimoria KY, McCarter MD, et al. Effect of body mass index on short-term outcomes after colectomy for cancer. J Am Coll Surg 2009;208:53–61.
- Nitori N, Hasegawa H, Ishii Y, et al. Impact of visceral obesity on short-term outcome after laparoscopic surgery for colorectal cancer: a single Japanese center study. Surg Laparosc Endosc Percutan Tech 2009;19:324–7.
- Park JS, Choi GS, Jang YS, et al. Influence of obesity on the serum carcinoembryonic antigen value in patients with colorectal cancer. Cancer Epidemiol Biomarkers Prev 2010;19:2461–8.
- Poulsen M, Ovesen H. Is laparoscopic colorectal cancer surgery in obese patients associated with an increased risk? Short-term results from a single center study of 425 patients. J Gastrointest Surg 2012;16:1554–8.
- Sakamoto K, Niwa S, Tanaka M, et al. Influence of obesity on the short-term outcome of laparoscopic colectomy for colorectal cancer. J Minim Access Surg 2007;3:98–103.
- Singh A, Muthukumarasamy G, Pawa N, et al. Laparoscopic colorectal cancer surgery in obese patients. Colorectal Dis 2011;13:878–83.
- Tsujinaka S, Konishi F, Kawamura YJ, et al. Visceral obesity predicts surgical outcomes after laparoscopic colectomy for sigmoid colon cancer. Dis Colon Rectum 2008;51:1757–65; discussion 65–7.
- Yamamoto N, Fujii S, Sato T, et al. Impact of body mass index and visceral adiposity on outcomes in colorectal cancer. Asia Pac J Clin Oncol 2012;8:337–45.
- Park JW, Lim SW, Choi HS, et al. The impact of obesity on outcomes of laparoscopic surgery for colorectal cancer in Asians. Surg Endosc 2010;24:1679–85.
- Sarkanen JR, Kaila V, Mannerstrom B, et al. Human adipose tissue extract induces angiogenesis and adipogenesis in vitro. Tissue Eng Part A 2012;18:17–25.
- Chen J, Katsifis A, Hu C, et al. Insulin decreases therapeutic efficacy in colon cancer cell line HT29 via the activation of the PI3K/Akt pathway. Curr Drug Discov Technol 2011;8:119–25.
- Guiu B, Petit JM, Bonnetain F, et al. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut 2010;59:341–7.
- Ghiringhelli F, Vincent J, Guiu B, et al. Bevacizumab plus FOLFIRI-3 in chemotherapy-refractory patients with metastatic colorectal cancer in the era of biotherapies. Invest New Drugs 2012;30:758–64.
- Simkens LH, Koopman M, Mol L, et al. Influence of body mass index on outcome in advanced colorectal cancer patients receiving chemotherapy with or without targeted therapy. Eur J Cancer 2011;47:2560–7.
- Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988;37:1595–607.
- Matsuzawa Y, Funahashi T, Nakamura T. The concept of metabolic syndrome: contribution of visceral fat accumulation and its molecular mechanism. J Atheroscler Thromb 2011;18:629–39.
- Karelis AD. Metabolically healthy but obese individuals. Lancet 2008;372:1281–3.
- Stocks T, Lukanova A, Bjorge T, et al. Metabolic factors and the risk of colorectal cancer in 580,000 men and women in the metabolic syndrome and cancer project (Me-Can). Cancer 2010. Published Online First: 17 December 2010.
- Stocks T, Lukanova A, Johansson M, et al. Components of the metabolic syndrome and colorectal cancer risk; a prospective study. Int J Obes (Lond) 2008;32:304–14.
- Hu NC, Chen JD, Lin YM, et al. Stepwise relationship between components of metabolic syndrome and risk of colorectal adenoma in a Taiwanese population receiving screening colonoscopy. J Formos Med Assoc2011;110:100–8.
- Kim JH, Lim YJ, Kim YH, et al. Is metabolic syndrome a risk factor for colorectal adenoma? Cancer Epidemiol Biomarkers Prev 2007;16:1543–6.
- Tsilidis KK, Brancati FL, Pollak MN, et al. Metabolic syndrome components and colorectal adenoma in the CLUE II cohort. Cancer Causes Control 2010;21:1–10.
- Pelucchi C, Negri E, Talamini R, et al. Metabolic syndrome is associated with colorectal cancer in men. Eur J Cancer 2010;46:1866–72.
- Ahmed RL, Schmitz KH, Anderson KE, et al. The metabolic syndrome and risk of incident colorectal cancer.Cancer 2006;107:28–36.
- Liu CS, Hsu HS, Li CI, et al. Central obesity and atherogenic dyslipidemia in metabolic syndrome are associated with increased risk for colorectal adenoma in a Chinese population. Bmc Gastroenterol 2010;10:51.
- Ashbeck EL, Jacobs ET, Martinez ME, et al. Components of metabolic syndrome and metachronous colorectal neoplasia. Cancer Epidemiol Biomarkers Prev 2009;18:1134–43.
- Healy LA, Howard JM, Ryan AM, et al. Metabolic syndrome and leptin are associated with adverse pathological features in male colorectal cancer patients. Colorectal Dis 2012;14:157–65.
- Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr 2007;86:s836–42.
- Luo Z, Saha AK, Xiang X, et al. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci 2005;26:69–76.
- Schoen RE, Tangen CM, Kuller LH, et al. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst 1999;91:1147–54.
- Oh TH, Byeon JS, Myung SJ, et al. Visceral obesity as a risk factor for colorectal neoplasm. J Gastroenterol Hepatol 2008;23:411–17.
- Yamaji T, Iwasaki M, Sasazuki S, et al. Visceral fat volume and the prevalence of colorectal adenoma. Am J Epidemiol 2009;170:1502–11.
- Otake S, Takeda H, Suzuki Y, et al. Association of visceral fat accumulation and plasma adiponectin with colorectal adenoma: evidence for participation of insulin resistance. Clin Cancer Res 2005;11:3642–6.
- Yamamoto S, Nakagawa T, Matsushita Y, et al. Visceral fat area and markers of insulin resistance in relation to colorectal neoplasia. Diabetes Care 2010;33:184–9.
- Nam SY, Kim BC, Han KS, et al. Abdominal visceral adipose tissue predictsrisk of colorectal adenoma in both sexes. Clin Gastroenterol Hepatol 2010;8:443–50 e1– 2.
- Erarslan E, Turkay C, Koktener A, et al. Association of visceral fat accumulation and adiponectin levels with colorectal neoplasia. Dig Dis Sci 2009;54:862–8.
- Barb D, Williams CJ, Neuwirth AK, et al. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr 2007;86: s858–66.
- Shehzad A, Iqbal W, Shehzad O, et al. Adiponectin: regulation of its production and its role in human diseases.Hormones (Athens) 2012;11:8–20.
- Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence.Endocr Rev 2012;33:47–94.
- Xu XT, Xu Q, Tong JL, et al. Meta-analysis: circulating adiponectin levels and risk of colorectal cancer and adenoma. J Dig Dis 2011;12:234–44.
- An W, Bai Y, Deng SX, et al. Adiponectin levels in patients with colorectal cancer and adenoma: a meta-analysis.Eur J Cancer Prev 2012;21:126–33.
- Gulcelik MA, Colakoglu K, Dincer H, et al. Associations between adiponectin and two different cancers: breast and colon. Asian Pac J Cancer Prev 2012;13:395–8.
- Wei EK, Giovannucci E, Fuchs CS, et al. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J Natl Cancer Inst 2005;97:1688–94.
- Lukanova A, Soderberg S, Kaaks R, et al. Serum adiponectin is not associated with risk of colorectal cancer.Cancer Epidemiol Biomarkers Prev 2006;15:401–2.
- Nakajima TE, Yamada Y, Hamano T, et al. Adipocytokines as new promising markers of colorectal tumors: adiponectin for colorectal adenoma, and resistin and visfatin for colorectal cancer. Cancer Sci 2010;101:1286–91.
- Svobodova S, Topolcan O, Holubec L Jr, et al. Parameters of biological activity in colorectal cancer. Anticancer Res 2011;31:373–8.
- Ferroni P, Palmirotta R, Spila A, et al. Prognostic significance of adiponectin levels in non-metastatic colorectal cancer. Anticancer Res 2007;27:483–9.
- Gialamas SP, Petridou ET, Tseleni-Balafouta S, et al. Serum adiponectin levels and tissue expression of adiponectin receptors are associated with risk, stage, and grade of colorectal cancer. Metabolism 2011;60:1530–8.
- Debucquoy A, Haustermans K, Daemen A, et al. Molecular response to cetuximab and efficacy of preoperative cetuximab-based chemoradiation in rectal cancer. J Clin Oncol 2009;27:2751–7.
- Landskroner-Eiger S, Qian B, Muise ES, et al. Proangiogenic contribution of adiponectin toward mammary tumor growth in vivo. Clin Cancer Res 2009;15:3265–76.
- Man K, Ng KT, Xu A, et al. Suppression of liver tumor growth and metastasis by adiponectin in nude mice through inhibition of tumor angiogenesis and downregulation of Rho kinase/IFN-inducible protein 10/matrix metalloproteinase 9 signaling. Clin Cancer Res 2010;16:967–77.
- Moon HS, Liu X, Nagel JM, et al. Salutary effects of adiponectin on colon cancer: in vivo and in vitro studies in mice. Gut 2012 Published Online First: 26 June 2012.
- Byeon JS, Jeong JY, Kim MJ, et al. Adiponectin and adiponectin receptor in relation to colorectal cancer progression. Int J Cancer 2010;127:2758–67.
- Sun Y, Lodish HF. Adiponectin deficiency promotes tumor growth in mice by reducing macrophage infiltration.PLoS One 2010;5:e11987.
- Fujisawa T, Endo H, Tomimoto A, et al. Adiponectin suppresses colorectal carcinogenesis under the high-fat diet condition. Gut 2008;57:1531–8.
- Saxena A, Chumanevich A, Fletcher E, et al. Adiponectin deficiency: role in chronic inflammation induced colon cancer. Biochim Biophys Acta 2012;1822:527–36.
- Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996;334:292–5.
- van Dielen FM, van’t Veer C, Schols AM, et al. Increased leptin concentrations correlate with increased concentrations of inflammatory markers in morbidly obese individuals. Int J Obes Relat Metab Disord2001;25:1759–66.
- Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology 2010;138:2029–43 e10.
- Kelesidis T, Kelesidis I, Chou S, et al. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med 2010;152:93–100.
- Wallace AM, Sattar N, McMillan DC. Effect of weight loss and the inflammatory response on leptin concentrations in gastrointestinal cancer patients. Clin Cancer Res 1998;4:2977–9.
- Arpaci F, Yilmaz MI, Ozet A, et al. Low serum leptin level in colon cancer patients without significant weight loss.Tumori 2002;88:147–9.
- Stattin P, Palmqvist R, Soderberg S, et al. Plasma leptin and colorectal cancer risk: a prospective study in Northern Sweden. Oncol Rep 2003;10:2015–21.
- Stattin P, Lukanova A, Biessy C, et al. Obesity and colon cancer: does leptin provide a link? Int J Cancer2004;109:149–52.
- Tamakoshi K, Toyoshima H, Wakai K, et al. Leptin is associated with an increased female colorectal cancer risk: a nested case-control study in Japan. Oncology 2005;68:454–61.
- Ho GY, Wang T, Gunter MJ, et al. Adipokines linking obesity with colorectal cancer risk in postmenopausal women.Cancer Res 2012;72:3029–37.
- Tutino V, Notarnicola M, Guerra V, et al. Increased soluble leptin receptor levels are associated with advanced tumor stage in colorectal cancer patients. Anticancer Res 2011;31:3381–3.
- Wang D, Chen J, Chen H, et al. Leptin regulates proliferation and apoptosis of colorectal carcinoma through PI3K/Akt/mTOR signalling pathway. J Biosci 2012;37:91–101.
- Kumor A, Daniel P, Pietruczuk M, et al. Serum leptin, adiponectin, and resistin concentration in colorectal adenoma and carcinoma (CC) patients. Int J Colorectal Dis 2009;24:275–81.
- Aloulou N, Bastuji-Garin S, Le Gouvello S, et al. Involvement of the leptin receptor in the immune response in intestinal cancer. Cancer Res 2008;68:9413–22.
- Uddin S, Bavi PP, Hussain AR, et al. Leptin receptor expression in Middle Eastern colorectal cancer and its potential clinical implication. Carcinogenesis 2009;30:1832–40.
- Endo H, Hosono K, Uchiyama T, et al. Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut 2011;60:1363–71.
- Nakajima A, Endo H, Hosono K, et al. Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut 2011;60:1363–71.
- Aparicio T, Guilmeau S, Goiot H, et al. Leptin reduces the development of the initial precancerous lesions induced by azoxymethane in the rat colonic mucosa. Gastroenterology 2004;126:499–510.
- Aparicio T, Kotelevets L, Tsocas A, et al. Leptin stimulates the proliferation of human colon cancer cells in vitro but does not promote the growth of colon cancer xenografts in nude mice or intestinal tumorigenesis in Apc(Min/+) mice. Gut 2005;54:1136–45.
- Drew JE. Molecular mechanisms linking adipokines to obesity-related colon cancer: focus on leptin. Proc Nutr Soc2011:1–6.
- Chia VM, Newcomb PA, Lampe JW, et al. Leptin concentrations, leptin receptor polymorphisms, and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev 2007;16:2697–703.
- Moller DE, Flier JS. Insulin resistance—mechanisms, syndromes, and implications. N Engl J Med 1991;325:938–48.
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature2006;444:840–6.
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev1995;16:3–34.
- Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 2008;8:915–28.
- LeRoith D, Roberts CT Jr. The insulin-like growth factor system and cancer. Cancer Lett 2003;195:127–37.
- Donohoe CL, Doyle SL, Reynolds JV. Visceral adiposity, insulin resistance and cancer risk. Diabetol Metab Syndr2011;3:12.
- Endo H, Hosono K, Fujisawa T, et al. Involvement of JNK pathway in the promotion of the early stage of colorectal carcinogenesis under high-fat dietary conditions. Gut 2009;58:1637–43.
- Freier S, Weiss O, Eran M, et al. Expression of the insulin-like growth factors and their receptors in adenocarcinoma of the colon. Gut 1999;44: 704–8.
- Yavari K, Taghikhani M, Maragheh M Ghannadi, et al. Downregulation of IGF-IR expression by RNAi inhibits proliferation and enhances chemosensitization of human colon cancer cells. Int J Colorectal Dis 2010;25:9–16.
- Rinaldi S, Cleveland R, Norat T, et al. Serum levels of IGF-I, IGFBP-3 and colorectal cancer risk: results from the EPIC cohort, plus a meta-analysis of prospective studies. Int J Cancer 2010;126:1702–15.
- Renehan AG, Zwahlen M, Minder C, et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet 2004;363:1346–53.
- Schoen RE, Weissfeld JL, Kuller LH, et al. Insulin-like growth factor-I and insulin are associated with the presence and advancement of adenomatous polyps. Gastroenterology 2005;129:464–75.
- Soubry A, Il’yasova D, Sedjo R, et al. Increase in circulating levels of IGF-1 and IGF-1/IGFBP-3 molar ratio over a decade is associated with colorectal adenomatous polyps. Int J Cancer 2012;131:512–17.
- Yamaji T, Iwasaki M, Sasazuki S, et al. Gender difference in the association of insulin and the insulin-like growth factor axis with colorectal neoplasia. Int J Obes (Lond) 2012;36:440–7.
- Keku TO, Vidal A, Oliver S, et al. Genetic variants in IGF-I, IGF-II, IGFBP-3, and adiponectin genes and colon cancer risk in African Americans and Whites. Cancer Causes Control 2012;23:1127–38.
- Hoyo C, Murphy SK, Schildkraut JM, et al. IGF2R genetic variants, circulating IGF2 concentrations and colon cancer risk in African Americans and Whites. Dis Markers 2012;32:133–41.
- Wu Y, Brodt P, Sun H, et al. Insulin-like growth factor-I regulates the liver microenvironment in obese mice and promotes liver metastasis. Cancer Res 2010;70:57–67.
- Serino M, Luche E, Gres S, et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut 2012;61:543–53.
- John BJ, Abulafi AM, Poullis A, et al. Chronic subclinical bowel inflammation may explain increased risk of colorectal cancer in obese people. Gut 2007;56:1034–5.
- Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology 2011;140:1807–16.
- Cani PD, Osto M, Geurts L, et al. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 2012;3:279–88.
- Rocha C Santos, Lakhdari O, Blottiere HM, et al. Anti-inflammatory properties of dairy lactobacilli. Inflamm Bowel Dis 2012;18:657–66.
- DiBaise JK, Zhang H, Crowell MD, et al. Gut microbiota and its possible relationship with obesity. Mayo Clin Proc2008;83:460–9.
- Pearson JR, Gill CI, Rowland IR. Diet, fecal water, and colon cancer— development of a biomarker. Nutr Rev2009;67:509–26.
- Islam KB, Fukiya S, Hagio M, et al. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 2011;141:1773–81.
- Zhu Y, Luo T Michelle, Jobin C, et al. Gut microbiota and probiotics in colon tumorigenesis. Cancer Lett2011;309:119–27.
- McMichael AJ, Potter JD. Host factors in carcinogenesis: certain bile-acid metabolic profiles that selectively increase the risk of proximal colon cancer. J Natl Cancer Inst 1985;75:185–91.
- Everson GT, McKinley C, Kern F Jr. Mechanisms of gallstone formation in women. Effects of exogenous estrogen (Premarin) and dietary cholesterol on hepatic lipid metabolism. J Clin Invest 1991;87:237–46.
- Danese E, Montagnana M, Minicozzi AM, et al. The role of resistin in colorectal cancer. Clin Chim Acta2012;413:760–4.
- Lee YJ, Myung SK, Cho B, et al. Adiposity and the risk of colorectal adenomatous polyps: a meta-analysis. Cancer Causes Control 2011;22:1021–35.